With the emergence of cell and gene therapy and other gene editing technologies, interest in efficient and effective gene delivery systems has surged in recent years. Commonly used systems include adeno-associated viruses (AAV) and lentiviruses. While both are effective transfer vehicles, there are key differences in how they infect cells and deliver their genetic payload. Most notably, lentiviruses have broader host-cell tropism than AAVs and offer stable integration into the host cell, whereas AAV tropism is narrower and only offers transient or episomal expression. Consequently, lentiviruses are the most utilize viral delivery system in both research and gene therapy applications. Here, we discuss how lentiviruses have been engineered to deliver transgenes safely and how researchers today generate lentiviral preparations.
Cellular Transduction with Lentivirus
Lentiviruses are enveloped retroviruses containing a single-stranded RNA genome. They are derived from the Retroviridae family, with some of the best-studied lentiviruses stemming from HIV and FIV. They utilize host cell machinery to enter target cells and manufacture protein and RNA components that self-assemble into infectious particles. Lentiviruses can infect both dividing and non-dividing cells and stably integrate their cargo into the host cell’s genome.
Infection begins when the glycoprotein envelope of the virus binds to the cell. Upon entry into the host cell, uncoating occurs followed by reverse transcription, mediated by a viral reverse transcriptase. The resulting linear double-stranded DNA is imported into the nucleus and is inserted into the host cell’s genome. The integrated viral genome, or provirus, undergoes transcription and translation to produce viral proteins, including structural proteins, enzymes, and gene regulators. The proteins and RNA copies assemble, bud, and mature, producing new viral particles (Figure 1).
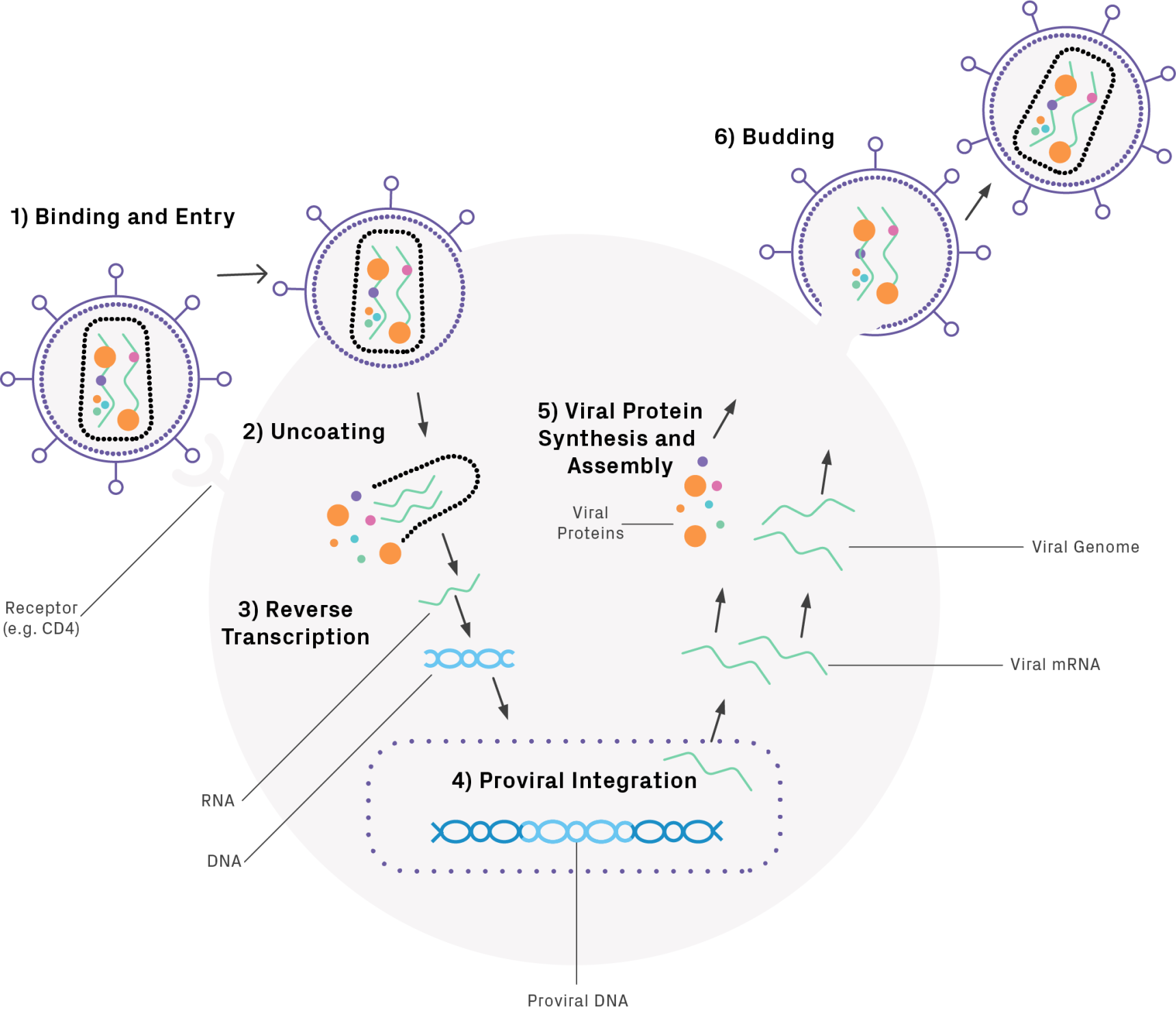
Lentivirus Production Overview
Using lentiviruses as gene delivery vehicles requires a series of steps to introduce the gene of interest (GOI) into a modified version of the viral genome and package it into a functional viral particle (Figure 2). First, the sequence of the GOI is designed and optimized for expression in the host organism. Consider the following recommendations when designing the construct.
- The transgene construct should be <9 kb for efficient lentiviral packaging
- Avoid internal poly A signals; they terminate transcription of GOI and other required genes
- Avoid toxic gene loads or unusual secondary structures
- Include a Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element (WPRE) sequence to enhance expression and facilitate functional titering (see ‘Titering Option’ section below)
The DNA is then synthesized and cloned into a lentiviral vector containing the necessary elements for genomic integration—such as long terminal repeat (LTR) regions—and expression (e.g., promoter). The plasmid DNA is amplified and transfected into a packaging cell line, such as HEK293T, which produces viral particles. Lastly, the concentration of viruses, or titer, is measured prior to use.
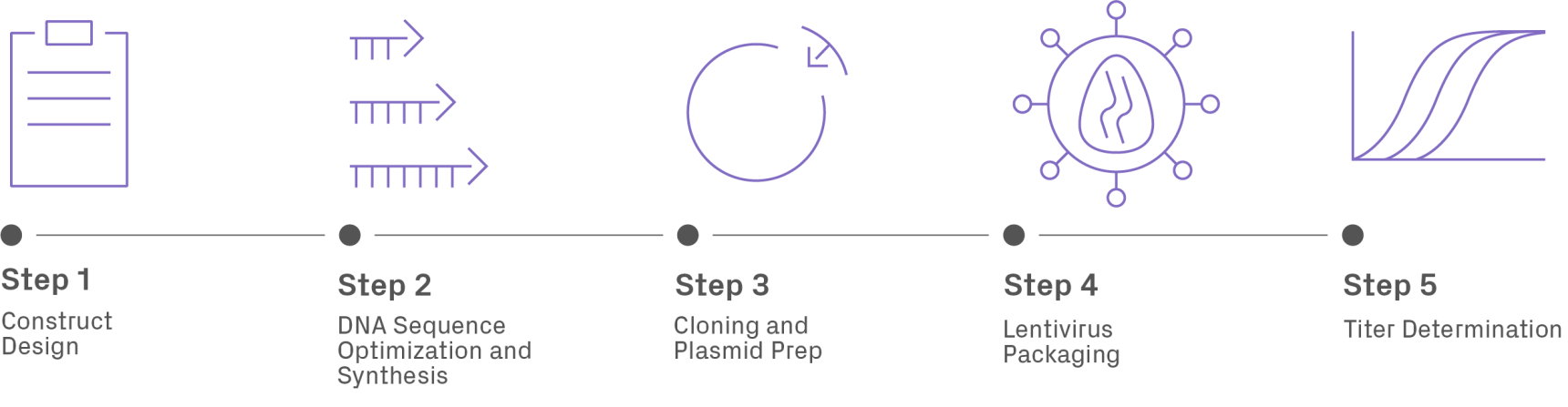
Current Lentiviral Vectors
As lentiviruses have grown in popularity for therapeutic applications, research and development has improved their efficacy and safety. Early lentiviral vectors (lentivectors) had the potential to generate replication-competent viruses, posing an infection risk to those working with them. Third generation vectors systems have segregated the integral pieces for gene transfer, packaging, and replication into separate plasmids that allow viral replication to proceed only when all necessary components are co-transfected into a cell line (see Figure 3). Additionally, the 3’ LTR of these vectors has been truncated to remove the tat gene, responsible for export of the virus from the host cell. This alteration makes these viruses replication incompetent and improves overall safety of the system. Fourth generation vectors have taken this a step further by also separating the gag (structural gene) and pol (reverse transcriptase/integrase) onto separate plasmids.
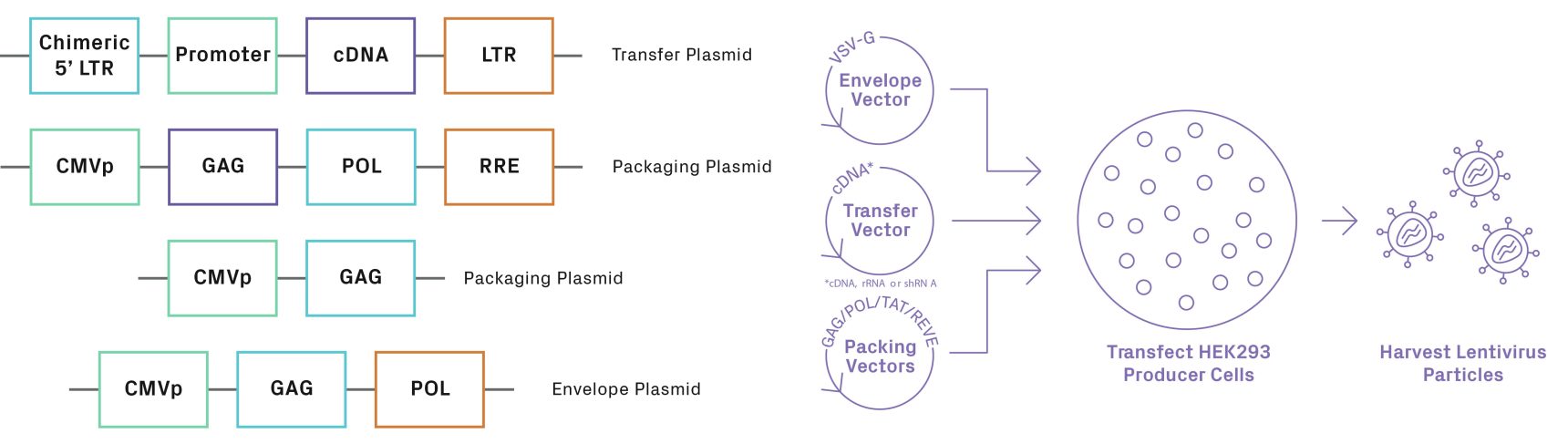
Lentiviral transfer vectors can drive transgene expression through different promoters, depending on the cell type that’s infected (see Table 1). Additionally, most vectors contain markers such as fluorescent proteins and/or antibiotic resistance genes for selection (e.g., puromycin). Lentiviral vectors offer a wide range of options for the co-expression of two transgenes, such as dual promoters or an internal ribosomal entry site (IRES). The IRES element, located between the two transgenes in the bicistronic vector, recruits ribosomes to initiate translation in a cap-independent manner. Other lentiviral vectors utilize the 2A ribosome skipping mechanism to generate multiple proteins from one transcript. The coding sequence for the 2A peptide, composed of 18 to 22 amino acids, is inserted between the two transgenes in the lentiviral construct. When the transcript is translated, the 2A peptide prevents normal peptide bond formation at its C terminus, cleaving the protein into two separate peptides.
Compare Plans
| PROMOTER | EXPRESSION LEVEL | APPLICATION |
|---|---|---|
| CMV | High | Commonly used in most cell lines (HeLa, HEK293, HT1080, etc.) |
| MSCV | High | Hematopoietic and stem cells |
| EF1α | Medium | Most cell types including primary cells and stem cells |
| PGK | Medium | Most cell types including primary cells and stem cells |
| UbC | Low | Most cell types including primary cells and stem cells |
Table 1. Lentivirus Transfer Vector Promoters and Markers
Titering Options for Lentiviral Preps
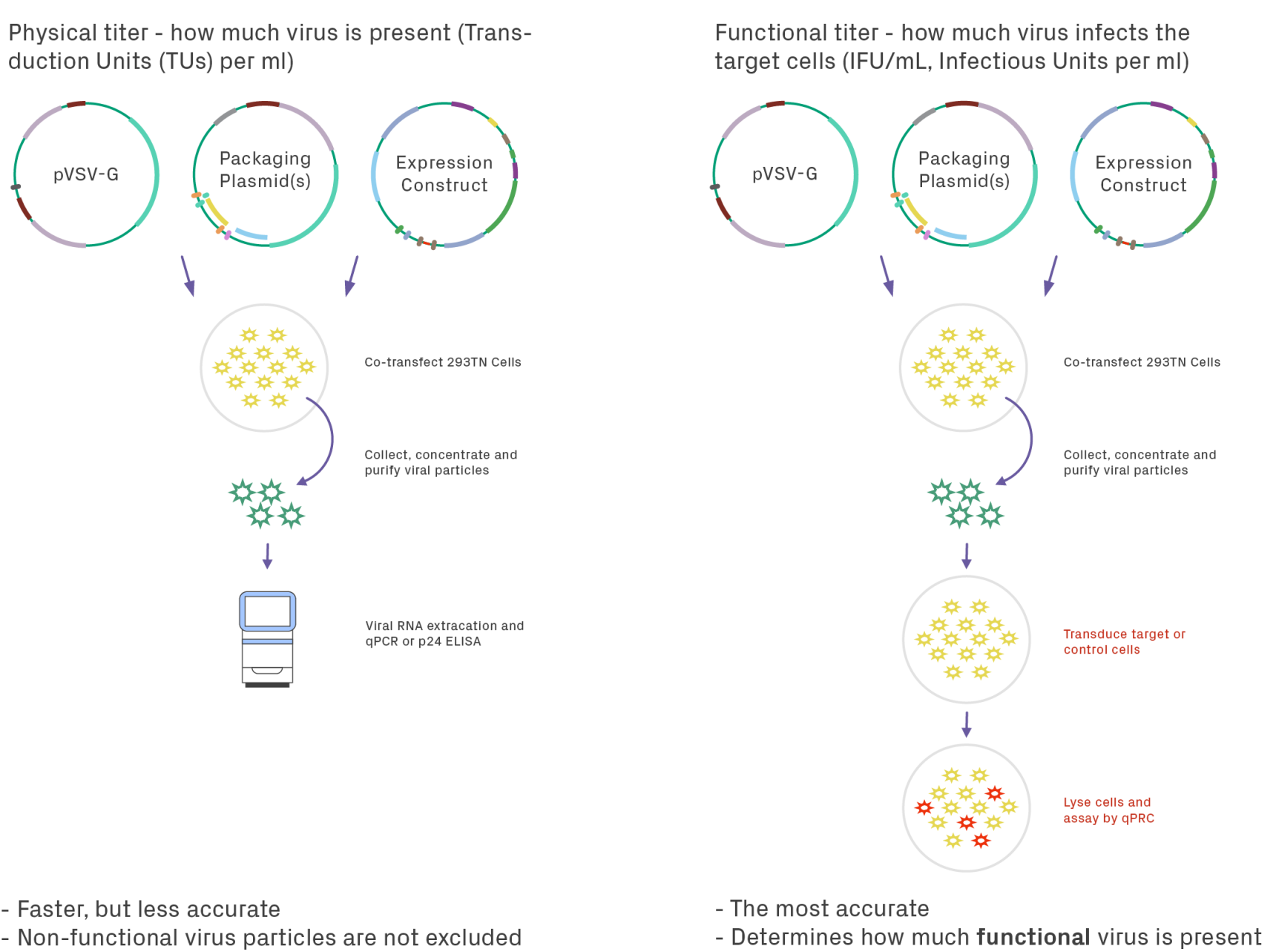
Measuring the titer of lentiviral preparations can be performed by two approaches: physical and functional. Physical titering measures the number of viral particles present in the sample, whereas functional titering measures the number of viral particles that can infect cells (Figure 4). Physical titering is typically performed via enzyme-linked immunoassay (ELISA) of the viral protein P24 and is reported in transduction units (TUs) per mL. This method, while fast, is not the most accurate because the functionality of the virus is not considered. Physical titers can be 10- to 1000-fold higher than the functional virus in a sample, which could impact the reproducibility of results between preps. With functional titering, a control cell line (often HT1080 cells) is transduced with the viral sample. The amount of infectious viral particles is determined by qPCR of the WPRE element, reported as infectious units (IFU) per mL.
Key Takeaways
- Lentiviruses are effective gene delivery systems, targeting a wide range of host cells and enabling stable expression of transgenes
- The latest generation of lentiviral vectors offer a high degree of biosafety with customizable promoters based on the research need
- Functional titering ensures the most accurate measurement of active viruses in a lentiviral preparation, improving downstream applications
Ready to use lentivirus as a gene delivery vector for your research? Learn how lentiviral packaging solutions can streamline the whole process.
References
1. Crespo-Barreda, M.M. Encabo-Berzosa, R. González-Pastor, P. Ortíz-Teba, M. Iglesias, J.L. Serrano, P. Martin-Duque. Chapter 11 – Viral and Nonviral Vectors for In Vivo and Ex Vivo Gene Therapies, Editor(s): Jeffrey Laurence,Translating Regenerative Medicine to the Clinic, Academic Press, 2016, Pages 155-177, ISBN 9780128005484.
2. Dong, W.; Kantor, B. Lentiviral Vectors for Delivery of Gene-Editing Systems Based on CRISPR/Cas: Current State and Perspectives. Viruses 2021, 13, 1288. https://doi.org/10.3390/v13071288
3. Gándara, Carolina, Valerie Affleck, and Elizabeth Ann Stoll. “Manufacture of third-generation lentivirus for preclinical use, with process development considerations for translation to good manufacturing practice.” Human gene therapy methods 29.1 (2018): 1-15
4. Global Lentiviral Vector Market – Industry Trends and Forecast to 2028. Data Bridge Market Research, June, 2021. https://www.databridgemarketresearch.com/reports/global-lentiviral-vector-market
5. Milone, Michael C., and Una O’Doherty. “Clinical use of lentiviral vectors.” Leukemia 32.7 (2018): 1529-1541.
6. Naso, Michael F, et al. “Adeno-Associated Virus (AAV) as a Vector for Gene Therapy.” BioDrugs : Clinical Immunotherapeutics, Biopharmaceuticals and Gene Therapy, Springer International Publishing, Aug. 2017.






